|
强生(JNJ)丙肝新药OLYSIO(simeprevir)获欧盟批准,联合其他药物,用于丙型肝炎病毒(HCV)基因型1和4慢性丙型肝炎(CHC)成人患者的治疗。该药将为欧洲患者提供一种新的三联疗法,同时将提供有史以来首个为期12周的无干扰素且不依赖利巴韦林及其他药物的治疗选择。
OLYSIO的获批,是基于II期COSMOS、3个关键III期QUEST-1、QUEST-2、PROMISE的数据。丙型肝炎(HCV)是一种血源性传染性肝脏疾病,若不及时治疗,可能对肝脏造成重大损害。
关于OLYSIO(simeprevir):
Simeprevir是新一代NS3/4A蛋白酶抑制剂,为每日一次的口服药物,由Medivir公司和杨森(Janssen)联合开发,用于治疗慢性丙型肝炎成年患者的代偿性肝病,包括各个阶段的肝纤维化,其工作原理是通过阻断蛋白酶,来抑制HCV在肝脏细胞中的复制。
simeprevir分别于2013年9月和11月获日本(在日本的商品名为Sovriad)和FDA批准,与聚乙二醇化干扰素和利巴韦林(ribavirin)联合用药,用于基因型-1慢性丙型肝炎病毒(HCV)感染者的治疗。
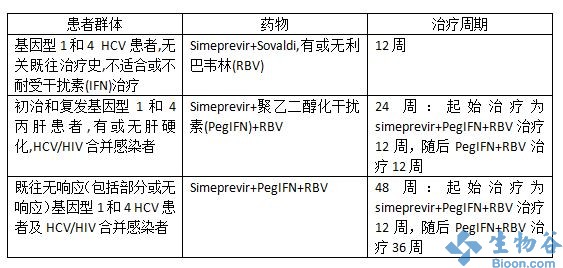
simeprevir是一种新的直接作用抗病毒药物,也是第二代蛋白酶抑制剂,给药方式为:simeprevir+聚乙二醇干扰素+利巴韦林联合治疗12周,随后进行聚乙二醇干扰素+利巴韦林治疗12周或36周。
阅读摘要原文
OLYSIO™ (simeprevir) receives marketing authorisation in the European union for the treatment of adults with hepatitis C genotype 1 and 4 infection
Simeprevir provides a new triple therapy treatment option, as well as the first ever 12-week interferon-free and ribavirin independent treatment regimen, in combination with sofosbuvir, for appropriate patients in Europe
BEERSE, BELGIUM [May 16, 2014] Janssen-Cilag International NV today announced that its next generation protease inhibitor (PI) OLYSIOTM (simeprevir) has been granted marketing authorisation by the European Commission (EC) for the treatment of adults with genotype 1 and 4 chronic hepatitis C (CHC), in combination with other medicinal products, which includes1:
This marketing authorisation represents a significant milestone in the development of new triple therapy hepatitis C (HCV) treatment options for genotype 1 and 4 patients. It also includes simeprevir as part of an all oral 12-week IFN-free direct-acting antiviral (DAA) regimen with or without RBV, in genotype 1 or 4 patients, who are intolerant to or ineligible for IFN treatment.[1]
“The EC marketing authorisation for simeprevir is a great milestone as it adds an important new treatment option for patients, demonstrating the continued role of triple therapy in the treatment of HCV. In addition, the introduction of an all oral, 12-week interferon-free treatment regimen provides a new option for sustained virologic response in HCV patients with genotypes 1 or 4 intolerant to or ineligible for interferon-based treatment,” said Thomas Stark, Medical Director, Janssen EMEA.
HCV represents a major global public health concern. There are an estimated nine million people[2] living with HCV in Europe which, if untreated, can cause severe damage to the liver, including cirrhosis and hepatocellular carcinoma (HCC). HCV represents a leading cause of liver transplantation in Europe.[3] Whilst the number of patients being newly diagnosed with HCV is declining, it takes approximately 20 – 30 years for symptoms to appear, with HCV cases expecting to peak between 2030 and 2035.[4],[5]
Dr Andrew Ustianowski, Chair of the British Viral Hepatitis Group and Consultant in Infectious Diseases at North Manchester General Hospital, commented: “The treatment environment in hepatitis C infection is evolving rapidly. Simeprevir is a well-tolerated and efficacious addition to our therapies against hepatitis C, and is a very welcome development for both those with genotype 1 and those with genotype 4.”
The EC marketing authorisation for simeprevir with PegIFN + RBV is based on a clinical trial programme involving three pivotal Phase 3 studies, with over 1000 patients. The trials, QUEST-1, QUEST-2[6] and PROMISE[7], explored the use of simeprevir in combination with PegIFN + RBV in treatment-naïve patients and patients who have relapsed after prior interferon-based treatment. All three studies met their primary endpoints and demonstrated that simeprevir, in combination with PegIFN + RBV, achieves significant sustained virological response rates when compared with PegIFN + RBV alone.
The EC marketing authorisation for the combination of simeprevir and sofosbuvir also contains results from the Phase 2 study, COSMOS, in treatment-naïve patients. This was based upon prior null responder and treatment-naïve patients.[8]
Simeprevir is taken once-daily for 12 weeks, with treatment-naïve and prior-relapser patients receiving pegylated interferon and ribavirin for 24 weeks, and for 48 weeks total by those shown to be prior non-responder patients (including partial and null responders)1. It is generally well tolerated, with the most common adverse events reported in clinical trials (incidence ≥ 5%) including nausea, rash, pruritus, dyspnoea, blood bilirubin increase and photosensitivity reaction.1
In March 2013, simeprevir was approved for the treatment of genotype 1 HCV in Japan, in Canada in September 2013, and the U.S. in November 2013, with the most recent approval occurring in Russia in March 2014. Following the EC marketing authorisation, it is anticipated that simeprevir will be available across a number of European union countries, in conjunction with reimbursement, in the second half of 2014.
About Simeprevir
Simeprevir is an NS3/4A protease inhibitor jointly developed by Janssen R&D Ireland and Medivir AB.
Janssen is responsible for the global clinical development of simeprevir and has exclusive, worldwide marketing rights, except in the Nordic countries. Medivir AB retains marketing rights for simeprevir in these countries under the marketing authorisation held by Janssen-Cilag International NV. Simeprevir was approved for the treatment of genotype 1 hepatitis C in September 2013 in Japan, in November 2013 in Canada and the U.S., and in March 2014 in Russia.
|


